Scientists have created a cow that produces human insulin in its milk. But the ethical debate surrounding genetic engineering remains as murky as ever.
Not so long ago, genetic engineering was little more than a science fiction fantasy, a “Twilight Zone” nightmare, or a “Seinfeld” punchline. (It’s a pig-man, Jerry!) But in the decades since the early days of cloning sheep, genetic engineering technology has grown vastly more sophisticated — and it’s rapidly transforming our relationship to livestock.
Last year the UK passed a law allowing genetic “precision breeding” technology to be used on any vertebrate animal. Early this month, the first dialysis patient to receive a pig kidney was discharged from a Boston hospital with a clean bill of health. And now, in one of the more imaginative applications of the technology, partnering scientists in Brazil and Illinois have successfully produced human insulin in the mammary gland of a cow — an advancement that may have dramatic implications for both the biomedical and the dairy industries, and the world’s growing population of diabetics.
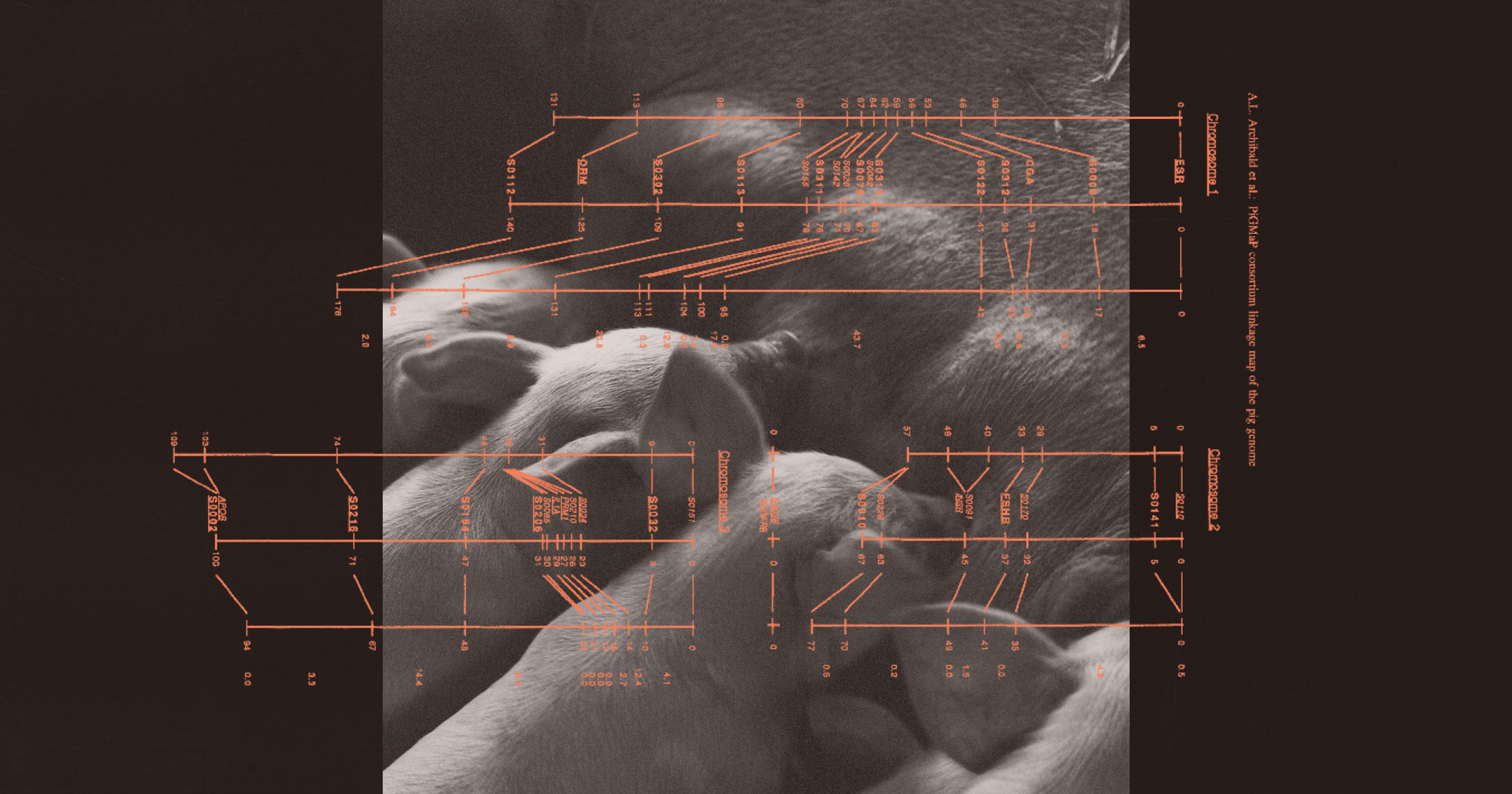
More than a decade ago, in an attempt to create a bio-based alternative to the expensive synthetic insulins that have saturated the market, researchers from Universidade de São Paulo and the University of Illinois inserted a gene for human proinsulin — the protein building blocks of insulin — into a cow embryo. (In a healthy human digestive system, this protein gets converted into insulin, which regulates the body’s sugar absorption.) That embryo was then transferred to the uterus of a regular cow back in Brazil.
Professor and researcher Matt Wheeler, lead author of the related study recently published in Biotechnology Journal, said that when the transgenic calf reached maturity, his team stimulated lactation using hormones after trying and failing to artificially inseminate her. What they found in her milk — not only human proinsulin but also actual insulin — was surprising.
The goal, Wheeler said, had been to extract proinsulin from the cow’s milk and purify it externally into insulin. But the cow skipped a step and did the purifying herself, wowing Wheeler and his team. “The mammary gland is designed as a protein factory, and it does a really, really good job,” he said. Whether it’s milk or insulin, mammaries are built to produce large volumes of nutrients — the perfect canvas for scientists like Wheeler who see those udders as an opportunity.
These findings have the potential to upend the current insulin industry and transform life with diabetes, one of the fastest growing global health crises — estimated to affect 783 million by 2045 — according to the International Diabetes Federation. Pharmaceutical insulin was once derived from the pancreases of cattle and pigs at slaughter, but as the need rose, drug companies began producing a synthetic insulin derived from bacteria and yeast. This is expensive and requires factories full of specialized equipment, Wheeler said over Zoom. He’d made his screen background a photograph of cows in Tanzania, taken during a work trip. “Whereas this beast that’s behind me,” he pointed to the cow grazing over his left shoulder, “is designed to do it essentially autonomously.”
At the University of California, Davis, researcher Elizabeth Maga has been busy with another kind of milk-based biomedical engineering, but in goats. Instead of insulin, Maga’s lab is interested in producing large volumes of lysozyme, an enzyme with properties that can treat and prevent diarrheal disease, which the World Health Organization notes is the third leading cause of death in children under five years old.
“The mammary gland is designed as a protein factory, and it does a really, really good job.”
Lysozyme is found in all mammal milk, Maga said, but is especially prevalent in human breast milk. Her team tends a small herd of transgenic goats that have received the human lysozyme gene and produce large quantities of the lysozyme-rich milk, which is currently being used in clinical trials with cancer patients in Los Angeles to help stem some of the gastrointestinal side effects of their treatment.
The capabilities of these transgenic animals could mean widespread improvements in quality of life for humans worldwide. If diabetics could someday eat or drink their medicine, for example, it would change countless lives. “Drinking a milkshake or a glass of milk, isn’t that a little more palatable than getting a shot every day?” Wheeler posed. “If I had to choose ice cream or give myself a shot, I pretty much know which one I would pick.”
But environmental advocates, wary of how unchecked genetic engineering could fundamentally disrupt the natural world, aren’t yet convinced. A spokesperson from the RSPCA (one of Britain’s largest animal welfare charities) called the UK’s new “precision breeding” law an “ill-judged policy.”
“Gene editing could be a huge step backwards for animals,” RSPCA rep David Bowles told The Guardian last year. He worried that “invasive procedures are needed to create each line of gene-edited mammals, there is no history of use for this powerful technology, and it can cause unintended changes to the genome, with unpredictable effects.”
So how far is too far? Wheeler asks himself that question regularly. “‘Can we?’ and ‘Should we?’ are two different things,” he said.
In the case of insulin-producing cows, he’s convinced that the humanitarian benefit outweighs any animal welfare concern. He said that the insulin production poses no more threat to a cow’s quality of life than standard dairy farming.
“As long as it was safe for our cows, it would be an honor to contribute to society and help people in such a monumental way.”
His team could have targeted other fluids that would be easier to collect from a cow, such as blood or urine. But they targeted milk in part because “the insulin that’s produced in the milk stays in the milk and doesn’t affect the cow.” It’s isolated to the mammary gland and doesn’t recirculate back into the animal, he said, noting that his team found “the blood levels of insulin were normal” within the transgenic cow. Studies have proven similar results in Maga’s goats.
While the moral and philosophical debate around genetic engineering remains far from settled, transgenic research may mean big changes for farmers. Anne Huebner, a Wisconsin dairy farmer and mother of four, heard about Wheeler’s insulin-producing cow through a friend with a diabetic child. Huebner reached out via LinkedIn to learn more, and the two spoke about Wheeler’s dreams for the future — one in which dairy farmers may someday raise herds for insulin just like milk. And while the “pharmaceutical glass of milk” Wheeler envisions is still a long way out, pending many more rounds of research and clinical trials, the next generation of dairy farmers will be paying close attention.
“A 50-cow dairy herd in Wisconsin is an endangered species,” Wheeler said. “But maybe you have these farms that have 50 cows making insulin. Their job is to make wholesome meat, milk, and fiber, that’s what farmers do. This is just another example of a value-added product.”
Because milk prices are so erratic, Huebner said that she and her husband are focused on creating a “viable business plan” that would allow them to continue farming even through fluctuations in the milk market. Wheeler’s vision of drinkable insulin produced by American dairy farms has a nice ring to it.
“God-willing — we’ve weathered the storms along the way and, as long as it was safe for our cows, it would be an honor to contribute to society and help people in such a monumental way,” Huebner said.
Over Zoom, Wheeler compared his transgenic cow to The Manhattan Project. While it’s certainly not as explosive, genetically engineered livestock does similarly push science to its outer limits and force us to tussle with questions about the sanctity of life.
It may turn out that the world isn’t ready for an insulin cow, he said. “But why not work toward an industry based on good, wholesome food that provides our medicines for us? Mother Nature is much better at making those things than we are. Let her help us.”